The lysophospholipid signal ing mediators Lysophosphatidic Acid and Sphingo sine one phosphate are vital regulators of neural improvement, modulating neural development, morphogene sis, and differentiation. Lysophospholipid signaling has become implicated in medi ating various physiological and pathological responses, as well as cancer progression, wound healing, angiogen esis, cardiovascular development, and, much more a short while ago, neural advancement, There exists sturdy evi dence that the two LPA and S1P are significant in early neural improvement, as mouse embryos that lack enzymes for S1P or LPA synthesis exhibit serious neural tube defects. Especially, mice with genetic deletion of Sphingosine kinases required for production of S1P created cranial neural tube defects due to enhanced apoptosis, decreased mitosis and subsequent thinning of your neu roepithelial progenitor cell layer, These information suggest that S1P mediates anti apoptotic and pro growth signal ing in ordinary neuroepithelial advancement.
Similarly, genetic deletion of Autotaxin, the enzyme liable for production of LPA while in the brain, yields embryonically lethal mice with neural tube defects. In these embryos, selleck inhibitor the neural tube fails to shut thoroughly and it is kinked, More, embryos lacking LPA exhibited asymmetric neu ral headfold, reflecting significant effusions with higher levels of apoptotic cells, These scientific studies demonstrate crucial and distinct roles of S1P and LPA in early neural growth. LPA and S1P receptors are expressed in neural progeni tors, neurons, and oligodendrocytes within the establishing and adult brain, and each LPA and S1P are generated by neurons, The biological consequences of lysophos pholipid signaling while in the nervous procedure are incompletely defined, but proof for several roles in neural progeni tors is emerging.
As talked about selleck above, there are actually clear roles for S1P and LPA in early neural tube development. Fur ther, LPA appears to regulate cortical neurogenesis by pro moting morphological alterations, survival, and differentiation, Eventually, S1P activity is implicated in mediating migration of neural progenitor cells toward websites of spinal damage, As a result, LPA and S1P regulate crit ical responses in neural progenitor cells that may be exploited to manipulate these cells in conventional pharma cological or cell based mostly therapeutics. LPA and S1P bind and activate cell surface G protein cou pled receptors to manage cell proliferation, dif 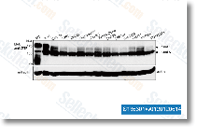 ferentiation, and morphological modifications, all of which may possibly contribute to their roles in regulating neural progen itor cell function.
ferentiation, and morphological modifications, all of which may possibly contribute to their roles in regulating neural progen itor cell function.
AChR signal
There is no boundary to the development of biotechnology and life sciences which are closely linked with human survival and indispensable to people's daily life.
